CARBONATION
Question: How does a baking soda volcano work? How does carbonation work? What is the difference between baking soda volcanoes and carbonation?
Hypothesis: We think that
|
Safety ProceduresDo NOT drink anything
Wear goggles Wear gloves |
Procedure: (1)Take the water bottle and fill it up half way with warm water.
|
Watch us in action:
|
|
(5) Take a soda bottle, shake it and observe the effect. You will see that it is a similar effect to the volcano.
|
The Science behind it
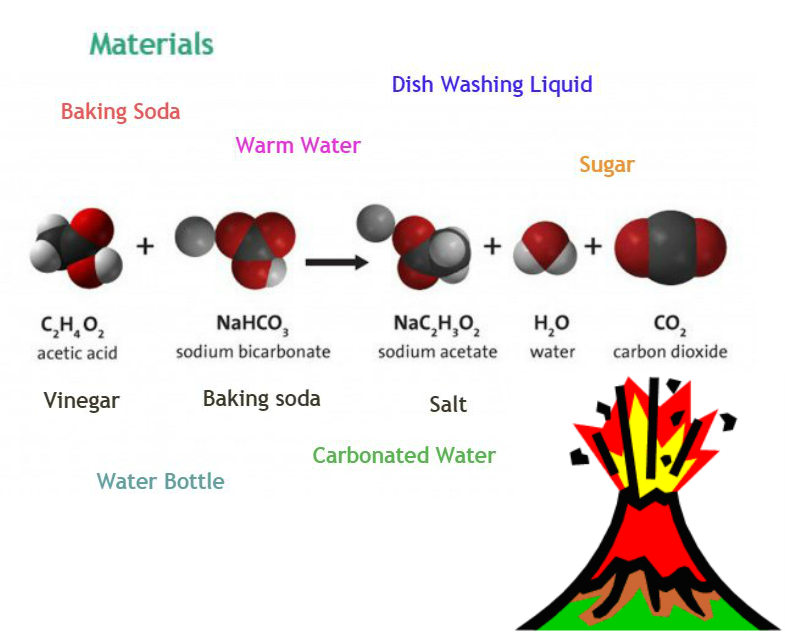
CH3COOH + NaHCO3 = H2O + C2O
The baking soda (sodium bicarbonate) is a base while the vinegar (acetic acid) is an acid. When they react together they form carbonic acid which is very unstable, it instantly breaks apart into water and carbon dioxide, which creates all the fizzing as it escapes the solution.
From http://www.sciencekids.co.nz/experiments/vinegarvolcano.html
The baking soda (sodium bicarbonate) is a base while the vinegar (acetic acid) is an acid. When they react together they form carbonic acid which is very unstable, it instantly breaks apart into water and carbon dioxide, which creates all the fizzing as it escapes the solution.
From http://www.sciencekids.co.nz/experiments/vinegarvolcano.html
Conclusions
About Author 1Hi My Name is Max. I am a catcher on the 11u baseball A team, My favorite Harry Potter characters are SNAPE (My Personal Favorite!) Dumbledore, and last , but definitely not least, Voldemort. Thomas and I could solve the Rubik's cube in in less than 30 seconds.
|
About Author 2 Hi my name is Thomas. I like a lot of things as a hobby:basketball,baseball, and solving the Rubik's cube. My favorite book is the Harry Potter Series and my favorite character is Harry because he was the chosen one to kill Voldemort and Voldemort try to kill Harry when he was a baby and Harry only got a scar! Haha
|
Comments
Question:
Hypothesis:
Materials:
Procedure:
Safety Procedure:
Observation:
Science Behind it:
Conclusion:
About Author:
Comments
Question:
Hypothesis:
Materials:
Procedure:
Safety Procedure:
Observation:
Science Behind it:
Conclusion:
About Author: