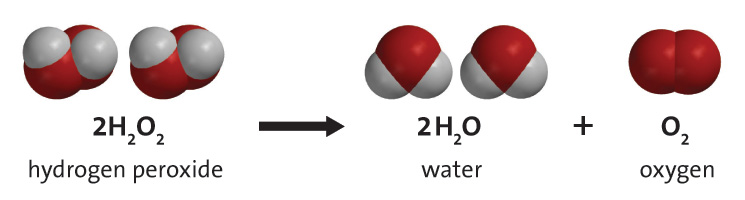
Question:
When we mix hydrogen peroxide, yeast, water and dishwashing soap, will the reaction change if we use 3% hydrogen peroxide versus 6% or 12% hydrogen peroxide?
Hypothesis:
We predict that if we increase the strength of the hydrogen peroxide to 12%, then the reaction will happen faster than it did with 3% hydrogen peroxide and more foam will be produced.
Materials:

- yeast
- dishwashing soap
- warm water
- plastic spoon
- funnel
- aluminum tray
- hydrogen peroxide
- food coloring
- mixing spoon
- empty water bottle
- measuring spoons
- measuring cup
- small bowl
- plastic disposable gloves
Special Needs:
- This experiment is messy so we need to use trays and also be close to a sink so you can clean up easily after this experiment.
Safety Procedures
- Be careful that the hydrogen peroxide doesn't touch your hair or clothes because it will bleach them.
- Don't look into the bottle when the reaction is happening because it will get into your eyes.
Procedure:
- Place empty water bottle in the center of aluminum tray
- Place funnel into water bottle
- Measure 1/2 cup of hydrogen peroxide and pour it through the funnel into the water bottle
- Measure 1 tablespoon of dish washing soap and pour it through the funnel into the water bottle
- Remove funnel from bottle and set aside
- Add 4 drops of food coloring to water bottle (optional; more food coloring will create brighter color)
- In a separate empty bowl or cup, measure and place 1 tablespoon of yeast
- Add 3 tablespoons of warm water to the yeast
- Stir mixture gently
- Place funnel back into the water bottle
- Add mixture to the water bottle
- Remove funnel quickly
- Stand back and watch the results!
The Science behind it
The yeast acted as a catalyst (helper) to break apart the oxygen and hydrogen peroxide. When the oxygen was free it filled lots of tiny bubbles from the liquid soap. These soap bubbles that filled with oxygen created the foam. This was also an exothermic reaction, which means it created heat. If you feel the bottle you will notice that it is warm.
Conclusions
When we increased the strength of the hydrogen peroxide to 12%, then the reaction happened faster than it did with 3% hydrogen peroxide and more foam was produced. So we were correct. The reaction time changed.
CaitlinHi! My name is Caitlin and I am 10 years old. I love to ice skate, dance, and do gymnastics. I figure skate, do ballet and jazz. I have a new puppy named Midnight. One day I would like to work with animals. I also love to bake. My favorite things to bake are apple pie and chocolate chip cookies. I love to experiment with baking and science!
|
MeaganHi! My name is Meagan and I am 10 years old. I love to dance and do gymnastics. When I have free time I love to bake and paint. I also like to do science experiments in my kitchen with anything I can find! I also love to care for babies and my little cousins. I just got a little kitten named Duchess. Someday I would like to be a vet.
|
GracieI am 10 years old. My favorite sport is baseball and I love to read Marvel and DC comics. My favorite color is purple and
I love to dance. I wanted to do this experiment for a long time. In all the past science fairs, I never got to see it in action, and I have alwasy wanted to. Now I get to see the science behind it. (Also, it reminds me of cupcake frosting!) |